

Pits of stone fruits (peaches, apricots – pits ground up).When ingested in LARGE quantities or NOT PREPARED correctly.Don’t confuse Acetone version with Acetonitrile version!!.Its metabolism creates inorganic cyanide.
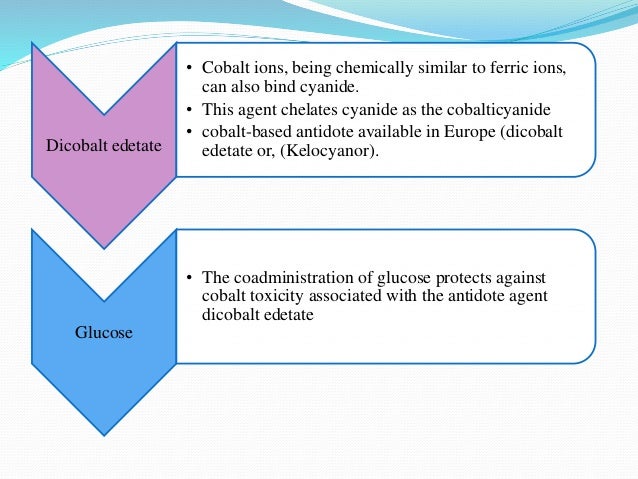
Acetonitrile is a industrial solvent and may be in cosmetics.Important example: False-Fingernail Remover (Acetonitrile-containing, NOT acetone-containing variety).Industrial sources may contain cyanide or cyanogenic compounds (like metal cleaning solutions).Young children at greater risk then adults of this exposure.Should be considered in any patient with smoke inhalation who is unstable, is acidotic, and/or had loss of consciousness (so don’t just consider carbon monoxide).Major source of exposure for both adults and children.Sources of Acute Cyanide Poisoning in Children Bitter-Almond odor may be appreciated (from un-metabolized cyanide).“Cherry-red” discoloration of the skin (lots of oxygen in the blood).Physical exam ( and board-style questions) may reveal:.Exposure to high concentrations of cyanide can lead to death within seconds to minutes.Severe poisoning can be rapidly fatal and there may not be a progression from “early” to “late” symptoms.Seizures, Coma, Death (the classic Toxic conclusion).Acute poisoning often leads symptoms related to end organs’ inability to generate ATP / being deprived of oxygen.Higher metabolic demands (typically require great oxygen consumption).Higher respiratory rates (greater inhalation exposure).Children are more at risk to toxicity (in general), because of:.Depend heavily on timing of exposure and lab draw, route of exposure, and specific cyanide compound.Treatment should be based on presumptive diagnosis.Toxicity can occur in even small doses.Shifts cells from aerobic to anaerobic metabolism.Inactivates cytochrome oxidase in mitochondria electron transport chain.


 0 kommentar(er)
0 kommentar(er)
